Introducing
iDose® TR (travoprost intracameral implant) 75 mcg
iDose TR is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).
iDose TR is the first-of-its-kind, long duration, anchored intracameral procedural pharmaceutical therapy for the treatment of OAG or OHT. It delivers continuous prostaglandin analog therapy directly into the anterior chamber to help with long-term IOP control with proven safety and patient tolerability.

24/7 Adherence with iDose TR
iDose TR is a paradigm-changing treatment.
Designed to deliver continuous therapeutic levels of a proprietary formulation of travoprost intracamerally
Demonstrated long-term IOP control in two robust pivotal trials
Provides proven safety and patient tolerability
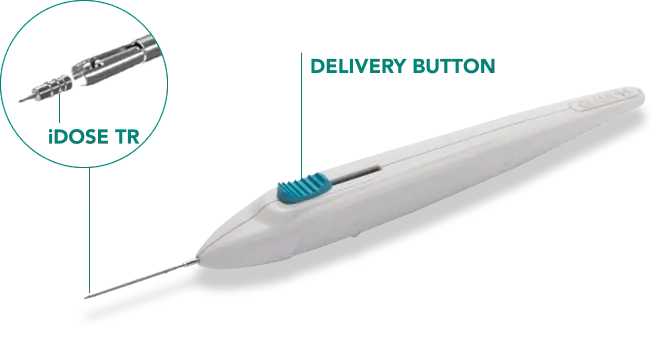
Unprecedented Technology
iDose TR is composed of a biocompatible intracameral implant preloaded in a sterile, single-dose inserter.
- Administers therapeutic levels of a highly-concentrated travoprost formulation
- Proprietary membrane enables consistent, long-term elution performance
- Anchored into scleral tissue to keep implant fixed and stable
Clinically Proven with Foundational IOP Control
In two pivotal trials, iDose TR achieved the pre-specified primary efficacy endpoint (non-inferiority to topical timolol through 3 months). At 12 months:

of iDose TR subjects were completely free of IOP-lowering topical medication1
Enduring Safety Profile
The most commonly reported ocular adverse reactions (2% to 6%) were increases in intraocular pressure, iritis, dry eye, visual field defects, eye pain, ocular hyperemia, and reduced visual acuity.2

- No clinically significant endothelial cell loss
- No periorbital fat atrophy
- No serious corneal AEs
- No DSAEK
- No DMEK
Resources
iDose TR Resources
HCP Resources
Get the facts and figures on the latest data, technology, and more to start integrating iDose TR into your practice.
Access Support
Overcome complex insurance coverage and reimbursement challenges with coding and billing
Patient Resources
Deliver patients access to insurance coverage resources, patient advocacy resources, and more.
Contact Us Today to Integrate iDose TR Into Your Practice
Complete the form to get started with offering iDose TR to your patients.
Request More Info
"*" indicates required fields
References
- iDose TR Phase 3 Clinical Trials, data on file, Glaukos Corporation.
- iDose TR (travoprost intracameral implant) 75 mcg Prescribing Information. Glaukos Corporation. 2023.
